In Forming A Solution The Solute Always - What causes a solution to form? The simple answer is that the solvent and the. A strong electrolyte always forms a very concentrated solution and a weak. When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. Dissolves dissociates both dissolves and dissociates. In forming a solution, the solute always: In order to form a solution, the solute must be surrounded, or solvated, by the solvent.
A strong electrolyte always forms a very concentrated solution and a weak. The simple answer is that the solvent and the. In order to form a solution, the solute must be surrounded, or solvated, by the solvent. When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. What causes a solution to form? Dissolves dissociates both dissolves and dissociates. In forming a solution, the solute always:
In order to form a solution, the solute must be surrounded, or solvated, by the solvent. In forming a solution, the solute always: When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. What causes a solution to form? A strong electrolyte always forms a very concentrated solution and a weak. Dissolves dissociates both dissolves and dissociates. The simple answer is that the solvent and the.
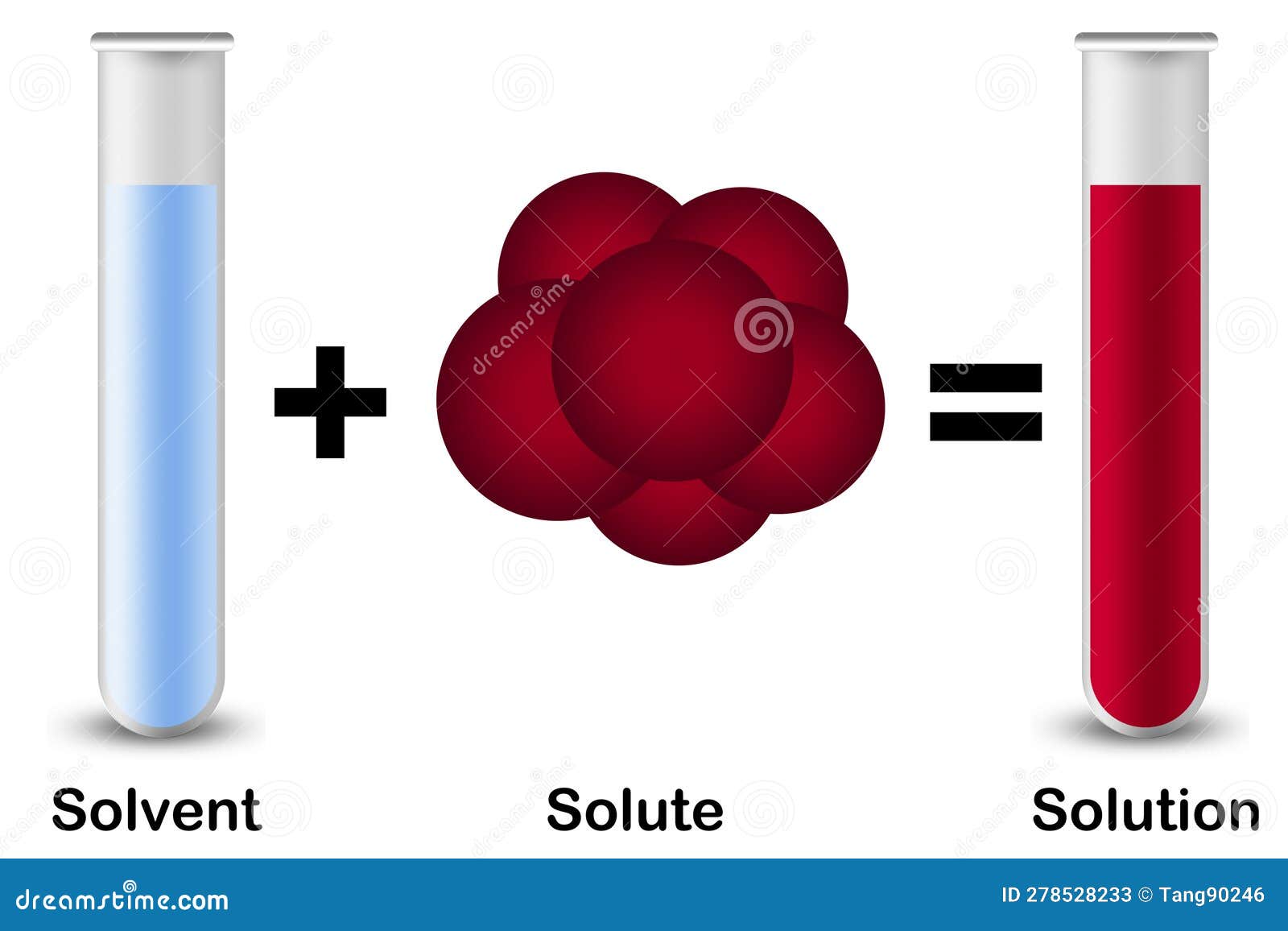
Solute, Solvent and Solution Isolated with Red Solute Stock
A strong electrolyte always forms a very concentrated solution and a weak. In order to form a solution, the solute must be surrounded, or solvated, by the solvent. In forming a solution, the solute always: When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. The simple answer is that the solvent and the.
Solute and Solvent Combinations — Overview & Examples Expii
A strong electrolyte always forms a very concentrated solution and a weak. What causes a solution to form? In forming a solution, the solute always: When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. Dissolves dissociates both dissolves and dissociates.
Solute Energy Education
A strong electrolyte always forms a very concentrated solution and a weak. The simple answer is that the solvent and the. In order to form a solution, the solute must be surrounded, or solvated, by the solvent. In forming a solution, the solute always: What causes a solution to form?
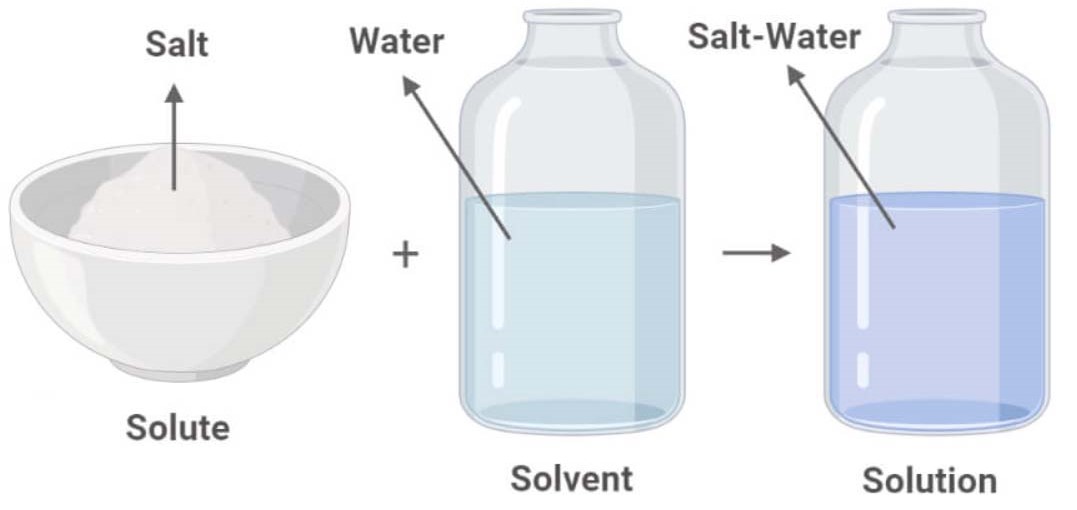
Solutions. Solubility homogeneous mixture. Solute, solvent and solution
The simple answer is that the solvent and the. What causes a solution to form? When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. In order to form a solution, the solute must be surrounded, or solvated, by the solvent. In forming a solution, the solute always:
In a solution, is the solute always a solid.
In order to form a solution, the solute must be surrounded, or solvated, by the solvent. When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. The simple answer is that the solvent and the. In forming a solution, the solute always: Dissolves dissociates both dissolves and dissociates.
Solute In Science
When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. What causes a solution to form? Dissolves dissociates both dissolves and dissociates. A strong electrolyte always forms a very concentrated solution and a weak. The simple answer is that the solvent and the.
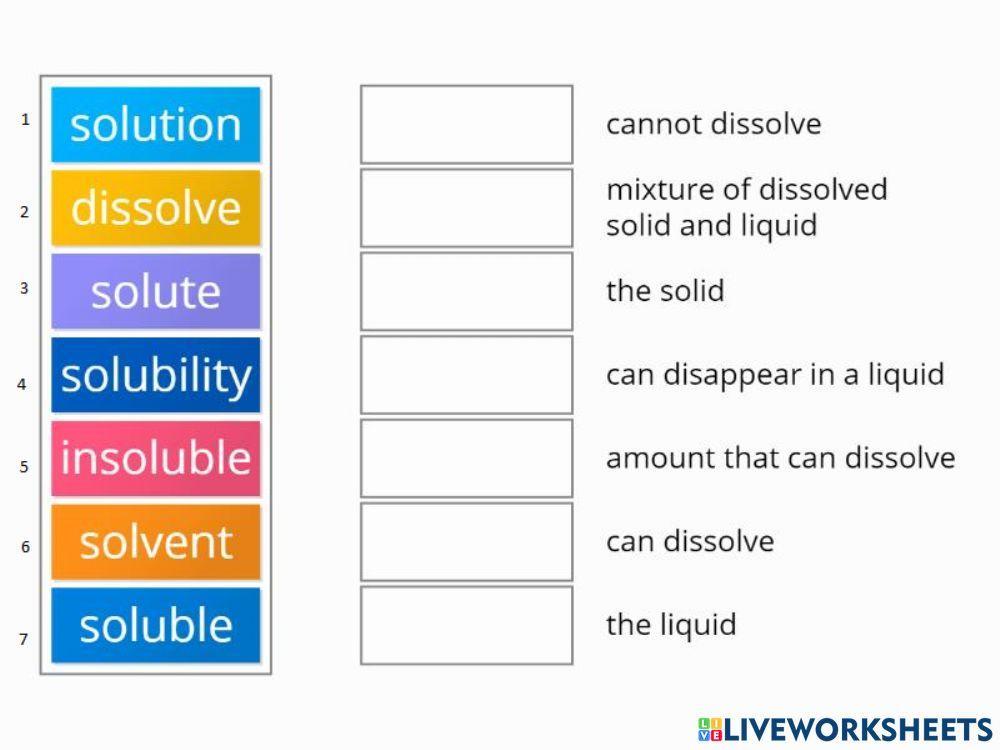
Solute vs. Solvent 5 Key Differences, Pros & Cons, Examples
In order to form a solution, the solute must be surrounded, or solvated, by the solvent. Dissolves dissociates both dissolves and dissociates. The simple answer is that the solvent and the. When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. A strong electrolyte always forms a very concentrated solution and a weak.
Understanding the Solute and Solvent Relationship
What causes a solution to form? When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. A strong electrolyte always forms a very concentrated solution and a weak. In forming a solution, the solute always: Dissolves dissociates both dissolves and dissociates.
Difference Between Solvent and Solute Definition, Properties, Examples
In order to form a solution, the solute must be surrounded, or solvated, by the solvent. When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. What causes a solution to form? The simple answer is that the solvent and the. A strong electrolyte always forms a very concentrated solution and a weak.
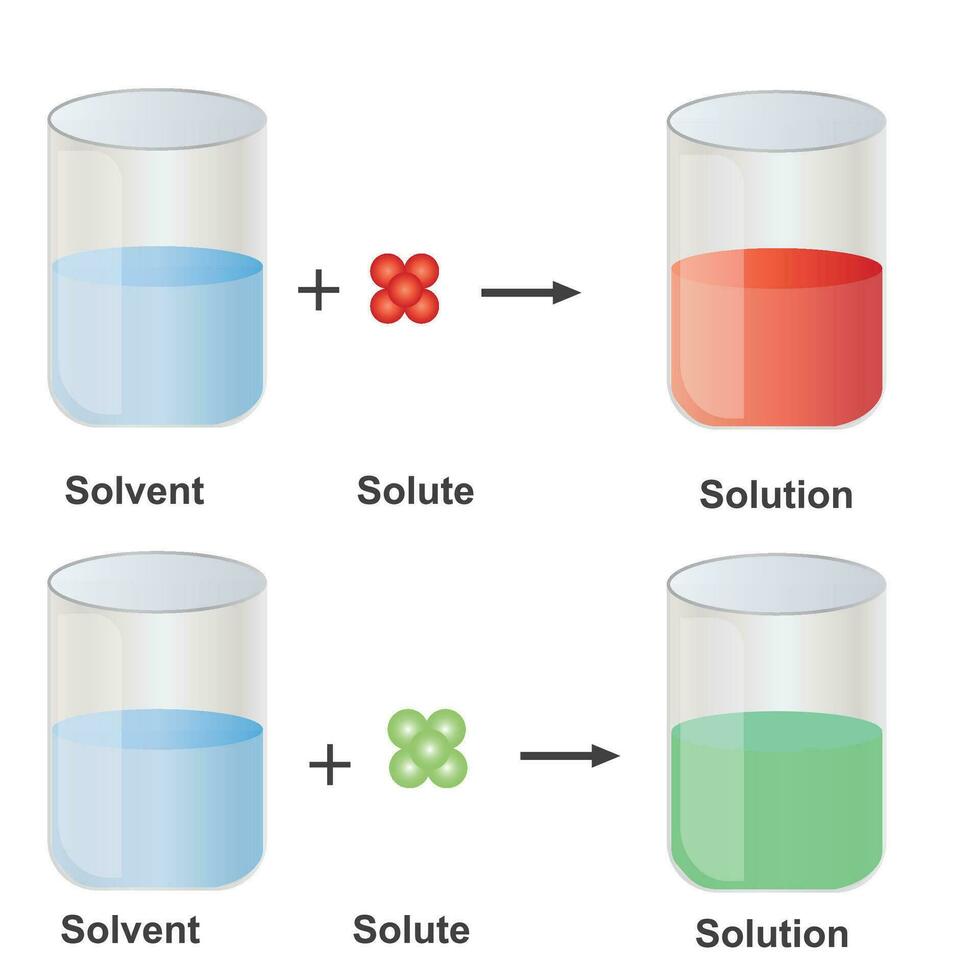
Examples Of Solute, Solvent And Solution Examples Of Solute, 44 OFF
In forming a solution, the solute always: In order to form a solution, the solute must be surrounded, or solvated, by the solvent. The simple answer is that the solvent and the. Dissolves dissociates both dissolves and dissociates. When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the.
A Strong Electrolyte Always Forms A Very Concentrated Solution And A Weak.
In forming a solution, the solute always: What causes a solution to form? In order to form a solution, the solute must be surrounded, or solvated, by the solvent. When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the.
Dissolves Dissociates Both Dissolves And Dissociates.
The simple answer is that the solvent and the.